- Lunar-synchronizated spawning mechanism in coral reef fishes
- Hormonal regulation of vitellogenesis
Lunar-synchronizated spawning mechanism in coral reef fishes
 Many fish species utilize cues from the moon for synchrony of growth, migration and gamete release. Since there are many adaptative strategies for lunar synchrony, it is considered that the cues utilized by the fish are different among species. Upon the earth, the moon exerts cyclic environmental changes, which may be due to intensity of moonlight, time of moonrise, relative to the solar cycle, and/or pattern of movement of the moon across the night sky. The earth-moon-sun gravitational forces (tidal forces), or the subtle alternations in the earth's geophysical forces that occur as a result of the changing position of the earth relative to the moon and the sun may not be excluded from perception of the lunar. it is expected that the fish can utilize such changes on the earth.
Many fish species utilize cues from the moon for synchrony of growth, migration and gamete release. Since there are many adaptative strategies for lunar synchrony, it is considered that the cues utilized by the fish are different among species. Upon the earth, the moon exerts cyclic environmental changes, which may be due to intensity of moonlight, time of moonrise, relative to the solar cycle, and/or pattern of movement of the moon across the night sky. The earth-moon-sun gravitational forces (tidal forces), or the subtle alternations in the earth's geophysical forces that occur as a result of the changing position of the earth relative to the moon and the sun may not be excluded from perception of the lunar. it is expected that the fish can utilize such changes on the earth.
We have demonstrated that rabbitfishes are restrict lunar-synchronized spawners. The gonad of each species develops toward and the mature gametes released at the specific lunar phase (see figure). The seagrass rabbitfish and the spiny rabbitfish spawn on or around the new moon, while the synchronous spawning of the golden rabbitfish and the forktail rabbitfish occurs around the first quarter moon and the last quarter moon, respectively. Using these rabbitfishes, we try to clarify physiological mechanism of lunar-shynchronized spawning.
Since lunar-shynchronized activity in fishes is biological rhythm, we think that our studies are categorized as chronobiology. Our research interests goes to what kind of the cue(s) from the moon are perceived by the fish and how it is conveyed as endogenous stimuli in endocrine organs in the fish. Recently, we demonstrated that melatonin which is produced in the pineal organ and the retina changes with not only daylignt but also moonlight. It is known that melaonin decreases during daytime and increases during night time. In addition to such daily change, melatonin increased around the new moon (dark night) and decreased around the full moon (bright night). This may mean that the fish can percieve change in moonlight intensity and utilize it for exertion of synchrony.
Related publications
- Sugama, N., Park, J.G., Park, Y.J., Takeuchi, Y., Kim, S.J. and Takemura, A. (2008) Moonlight affects nocturnal Period2 transcript levels in the pineal gland of the reef fish Siganus guttatus. Journal of Pineal Research, in press.
- Pisingan, R.S. and Takemura, A. Apparent semilunar spawning rhythmicity in a brackish cardinalfish, Apogon amboinensis. Journal of Fish Biology, 70: 1412-1422. [abstract].
- Park, Y.J., Park, J.G., Jeong, H.B., Takeuchi, Y., Kim, S.J., Lee, Y.D. and Takemura, A. Expression of the melatonin receptor Mel1c in neural tissues of the reef fish Siganus guttatus. Comparative Physiology and Biochemistry, 147A: 103-111. [abstract]
- Park, J.G., Park, Y.J., Sugama, N., Kim, S.J. and Takemura, A. Molecular cloning and diurnal variations of the Period gene in a reef fish Siganus guttatus. Journal of Comparative Physiology A, 193: 403-411. [abstract]
- Park, Y.J., Park, J.G., Hiyakawa, N., Lee, Y.D., Kim, S.J. and Takemura, A. (2007). Diurnal and circadian regulation of a melatonin receptor, MT1, in the golden rabbitfish, Siganus guttatus. General and Comparative Endocrinology, 150: 253-162. [abstract]
- Ayson, F.G. and Takemura, A. (2007). mRNA expression patterns for GH, PRL, SL, IGF-I and IGF-II during altered nutritional status in rabbitfish, Siganus guttatus. General and Comparative Endocrinology, 150: 196-204. [abstract]
- Pisingan, R.S., Harnadi, L. and Takemura, A. (2006). Semilunar spawning periodicity in the brackish damsel, Pomacentrus taeniometopon Breeker. Fisheries Science, 72: 1256-1260. [abstract]
- Ayson, F.G. and Takemura, A. (2006). Daily expression patterns for mRNAs of GH, PRL, SL, IGF-I and IGF-II in juvenile rabbitfish, Siganus guttatus, during 24-hour light and dark cycles. General and Comparative Endocrinology, 149: 261-268. [abstract]
- Park, Y.J., Park, J.G., Kim, S.J., Lee, Y.D., Rahman, M.S. and Takemura, A. (2006). Melatonin receptor of a reef fish with lunar-related rhythmicity: cloning and daily variations. Journal of Pineal Research, 41: 166-174. [abstract]
- Park, Y.J., Takemura, A. and Lee, Y.D. (2006). Histological evidence of lunar-synchronized reproductive activity in the pencil-streaked rabbitfish, Siganus doliatus, the Chuuk Lagoon, Micronesia. Ichthyological Research, 53: 179-181. [abstract]
- Park, Y.J., Takemura, A. and Lee, Y.D. (2006). Annual and lunar-synchronized reproductive activity in two rabbitfish species in the Chuuk Lagoon, Micronesia. Fisheries Science, 72: 166-172. [abstract]
- Takemura, A., Ueda, S., Hiyakawa, N. and Nikaido, Y. (2006). A direct influence of moonlight intensity on changes in melatonin production by cultured pineal glands of the golden rabbitfish, Siganus guttatus. Journal of Pineal Research, 40: 236-241. [abstract]
- Takemura, A., Rahman, M.S., Nakamura, S., Park, Y.J. and Takano, K.(2004). Lunar cycles and reproductive activity in reef fishes with particular attention to rabbitfishes. Fish and Fisheries, 5: 317-328. [abstract]
- Takemura, A., Susilo, E.S., Rahman, M.S. and Morita, M. (2004). Perception and possible utilization of moonlight intensity for reproductive activities in a lunar-synchronized spawner, the golden rabbitfish. Journal of Experimental Zoology, 301A: 844-851. [abstract]
- Rahman, M.S., Kim, B.H., Takemura, A., Park, C.B. and Lee, Y.D. (2004). Effects of moonlight exposure on plasma melatonin rhythms in the seagrass rabbitfish, Siganus canaliculatus. Journal of Biological Rhythms, 19: 325-334. [abstract]
- Rahman, M.S., Kim, B.H., Takemura, A., Park, C.B. and Lee, Y.D. (2004). Influence of light-dark and lunar cycles on the ocular melatonin rhythms in the seagrass rabbitfish, a lunar-synchronized spawner. Journal of Pineal Research, 37: 122-128. [abstract]
- Rahman, M.S., Takemura, A., Park, Y.J., Takano, K. (2003). Lunar cycle in the reproductive activity in the forktail rabbitfish. Fish Physiology and Biochemistry, 28: 443-444. [abstract]
- Rahman, M.S., A. Takemura, S. Nakamura and K. Takano (2003). Rhythmic changes in testicular activity with lunar cycle in the forktail rabbitfish. Journal of Fish Biology, 62: 495-499. [abstract]
- Rahman, M.S., Morita, M., Takemura, A. and Takano, K. (2003). Hormonal changes in relation to lunar periodicity in the testis of the forktail rabbitfish, Siganus argenteus. General and Comparative Endocrinology, 131, 302-309. [abstract]
- Harahap, A.P., A. Takemura, S. Nakamura, M.S. Rahman, and K. Takano (2002). Lunar-synchronization in acquisition of sperm motility in the spiny rabbitfish, Siganus spinus (Linnaeus). Fisheries Science, 68: 706-708. [abstract]
- Rahman, M.S. A. Takemura and K. Takano (2002). Lunar synchronization of in vitro steroidogenesis by ovaries in the golden rabbitfish, Siganus guttatus (Bloch). General and Comparative Endocrinology, 125: 1-8. [abstract]
- Lee, Y.-D., Park, S.-H., Takemura, A. and Takano K. (2002). Histological observations of seasonal reproductive and lunar-related spawning cycles in the female honeycomb grouper, Epinephelus merra, in Okinawan water. Fisheries Science, 68: 872-877. [abstract]
- Rahman, M.S., A. Takemura and K. Takano (2001). Lunar-synchronization of testicular development and steroidogenesis in rabbitfish. Comparative Biochemistry and Physiology, 129B: 367-373. [abstract]
- Harahap, A.P., A. Takemura, S. Nakamura, M.S. Rahman, and K. Takano (2001). Histological evidence of lunar-synchronized gonadal maturation and spawning in the spiny rabbitfish, Siganus spinus (Linnaeus), around the Ryukyus. Fisheries Science, 67: 888-893. [abstract]
- Rahman M.S., A. Takemura and K. Takano (2000). Correlation between plasma steroid hormones and vitellogenin profiles and lunar periodicity in the female golden rabbitfish, Siganus guttatus (Bloch). Comparative Biochemistry and Physiology,127B:113-122. [abstract]
- Rahman, M.S., A. Takemura and K. Takano (2000). Lunar synchronization of testicular development and plasma steroid hormone profiles in the golden rabbitfish, Siganus guttatus (Bloch). Journal of Fish Biology, 57(4): 1065-1074. [abstract]
- Hoque, M.M., A. Takemura, M. Matsuyama, S. Matsuura and K. Takano (1999). Lunar spawning in Siganus canaliculatus. Journal of Fish Biology, 55: 1213-1222. [abstract]
- Fujita, T., A. Takemura and K. Takano (1997). Histological observations of annual reproductive and tidal spawning rhythm in the female porcupine fish Diodon holocanthus. Fisheries Science, 65: 715-720.
Hormonal regulation of vitellogenesis
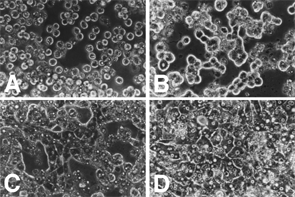 Vitellogenin (VTG) is one of female-specific proteins. VTG appears in the blood circulation of female fish starting vitellogenesis and of male or immature fish treated with estrogens (e.g. estradiol-17b, E2). This protein is a precoursor form of egg yolk and is synthesized in the liver under influence of E2. It is generally accepted that E2 is the most important steroid hormones to induce VTG in fish hepatocytes. However, direct or indirect requires of additional hormones and components in inducing VTG have been suggested in some teleost species such as rainbow trout, Japanese eel and Europian eel. Using tilapia and rabbitfish, we try to clarify physiological mechanism of vitellogenesis in fishes.
Vitellogenin (VTG) is one of female-specific proteins. VTG appears in the blood circulation of female fish starting vitellogenesis and of male or immature fish treated with estrogens (e.g. estradiol-17b, E2). This protein is a precoursor form of egg yolk and is synthesized in the liver under influence of E2. It is generally accepted that E2 is the most important steroid hormones to induce VTG in fish hepatocytes. However, direct or indirect requires of additional hormones and components in inducing VTG have been suggested in some teleost species such as rainbow trout, Japanese eel and Europian eel. Using tilapia and rabbitfish, we try to clarify physiological mechanism of vitellogenesis in fishes.
Related publications
- Kim, B.H., Takemura, A., Kim, S.J. and Lee, Y.D. (2003). Vitellogenin induction via androgens in primary cultures of tilapia hepatocytes. General and Comparative Endocrinology, 132: 248-255. [abstract]
- Kim, B.H. and Takemura, A. (2003). Culture conditions affect induction of vitellogenin synthesis by estradiol-17b in primary cultures of tilapia hepatocytes. Comparative Biochemistry and Biochemistry, 135B: 231-239. [abstract]
- Kim, B.H. and Takemura, A. (2002). In vitro vitellogenin synthesis in primary cultures of tilapia hepatocytes. Fisheries Science, 68: 123-131. [abstract]
- Kim, B.H., Takemura, A. and Nakamura, M. (2002). Comparison of in vitro vitellogenin synthesis among different nonylphenol products using primary cultures of tilapia hepatocytes. Fisheries Science, 68: 838-842. [abstract]
- Takemura, A. and Kim, B.H. (2001). Effects of estradiol-17b on in vitro and in vivo synthesis of two distinct vitellogenins in tilapia. Comparative Biochemistry and Physiology, 129A: 641-651. [abstract]
- Vijayan, M.M., Takemura, A. and Mommsen, T.P. (2001). Estradiol impairs hypo-osmoregulatory capacity in the euryhaline tilapia, Oreochromis mossambicus. American Journal of Physiology, 281: R1161-R1168. [abstract]
- Rahman, M.S., Takemura, A. and Takano, K. (2000). Annual changes in ovarian histology, plasma steroid hormones and vitellogenin in the female golden rabbitfish, Siganus guttatus. Bulletin of Marine Science, in press.
- Rahman, M.S., Takemura, A. and Takano, K. (2000). Correlation between plasma steroid hormones and vitellogenin profiles and lunar periodicity in the female golden rabbitfish, Siganus guttatus (Bloch). Comparative Biochemistry and Physiology, 127B: 113-122. [abstract]
- Takemura, A., Takeuchi, H., Teruya, T., Oka, M. and Kanematsu, M. (1999). Immunochemical estimation of reproductive activity in hatchery-reared female greater amberjack, Seriola dumerili, using skin mucus. Fisheries Science, 65: 792-793.
- Takemura, A. and Sin, D.H. (1999). Detection of vitellogenin in the sera of male tilapia inhabiting the Okukubi river, using an enzyme-linked immunosorbent assay (ELISA). Biological Magazine, Okinawa, 37: 1-7.
- Takemura, A. and Oka, M. (1998). Immunochemical sexing of living yellowfin tuna, Thunnas albacares (Bonnaterre), using a vitellogenin-like protein. Aquaculture Research, 29: 245-250.
- Fujita, T., Takemura, A. and Takano, K. (1998). Immunochemical detection of precursor proteins of yolk and vitelline envelope, and their annual changes in the blood of Diodon holocanthus. Journal of Fish Biology, 52; 1229-1240. [abstract]
- Takemura, A. and Teruya, T. (1997). Purification and partial characterization of the vitellogenin of coral trout, Plectropomus leopardas. Bulletin of Marine Science, 61(3): 791-800.
- Choi, C.Y., Chang, Y.J., Takemura, A. and Takano, K. (1996). Immunochemical properties of vitellogenin and egg yolk proteins in female fusilier, Caesio diagramma. Journal of Aquaculture, 9(1): 83-92.
- Choi, C.Y., Takemura, A. and Takano, K. (1996). Ovarian development and changes in serum vitellogenin during the annual reproductive cycle of the female fusilier, Caesio diagramma. Galaxea, 13: 35-45.
- Takemura, A., Takano, K. and Takahashi, H. (1995). The uptake of macromolecular materials in the hindgut of viviparous rockfish embryos. Journal of Fish Biology, 46: 485-493. [abstract]
- Choi, C.Y., Chang, Y.J. and Takemura, A. (1995). Purification of vitellogenin and egg yolk protein, and changes of vitellogenin concentration during the ovulation period in elkhorn sculpin, Alcichthys alcicornis. Journal of the Korean Fisheries Society, 28(6): 753-760.
- Takemura, A. (1994). Vitellogenin-like substance in the skin mucus of Oreochromis mossambicus. Fisheries Science, 60(6): 789-790.
- Takemura, A., Nunomura, W., Takano, K. and Hirai, H. (1993). Novel female protein with biological activities of C-reactive protein and lectin in a viviparous rockfish, Sebastes taczanowskii. The Journal of Experimental Zoology, 266: 188-194.
- Takemura, A., Hara, A. and Takano, K. (1991). Immunochemical identification and partial characterization of female-specific serum proteins in white-edged rockfish, Sebastes taczanowskii. Environmental Biology of Fishes, 30: 49-56.
- Quinitio, G.F., Takemura, A. and Goto, A. (1989). Ovarian development and changes in the serum vitellogenin levels in the river sculpin, Cottus hangiongensis, during an annual reproductive cycle. Bulletin of the Faculty of Fisheries, Hokkaido University, 40(4): 246-253.

 Many fish species utilize cues from the moon for synchrony of growth, migration and gamete release. Since there are many adaptative strategies for lunar synchrony, it is considered that the cues utilized by the fish are different among species. Upon the earth, the moon exerts cyclic environmental changes, which may be due to intensity of moonlight, time of moonrise, relative to the solar cycle, and/or pattern of movement of the moon across the night sky. The earth-moon-sun gravitational forces (tidal forces), or the subtle alternations in the earth's geophysical forces that occur as a result of the changing position of the earth relative to the moon and the sun may not be excluded from perception of the lunar. it is expected that the fish can utilize such changes on the earth.
Many fish species utilize cues from the moon for synchrony of growth, migration and gamete release. Since there are many adaptative strategies for lunar synchrony, it is considered that the cues utilized by the fish are different among species. Upon the earth, the moon exerts cyclic environmental changes, which may be due to intensity of moonlight, time of moonrise, relative to the solar cycle, and/or pattern of movement of the moon across the night sky. The earth-moon-sun gravitational forces (tidal forces), or the subtle alternations in the earth's geophysical forces that occur as a result of the changing position of the earth relative to the moon and the sun may not be excluded from perception of the lunar. it is expected that the fish can utilize such changes on the earth.  Vitellogenin (VTG) is one of female-specific proteins. VTG appears in the blood circulation of female fish starting vitellogenesis and of male or immature fish treated with estrogens (e.g. estradiol-17b, E2). This protein is a precoursor form of egg yolk and is synthesized in the liver under influence of E2. It is generally accepted that E2 is the most important steroid hormones to induce VTG in fish hepatocytes. However, direct or indirect requires of additional hormones and components in inducing VTG have been suggested in some teleost species such as rainbow trout, Japanese eel and Europian eel. Using tilapia and rabbitfish, we try to clarify physiological mechanism of vitellogenesis in fishes.
Vitellogenin (VTG) is one of female-specific proteins. VTG appears in the blood circulation of female fish starting vitellogenesis and of male or immature fish treated with estrogens (e.g. estradiol-17b, E2). This protein is a precoursor form of egg yolk and is synthesized in the liver under influence of E2. It is generally accepted that E2 is the most important steroid hormones to induce VTG in fish hepatocytes. However, direct or indirect requires of additional hormones and components in inducing VTG have been suggested in some teleost species such as rainbow trout, Japanese eel and Europian eel. Using tilapia and rabbitfish, we try to clarify physiological mechanism of vitellogenesis in fishes.